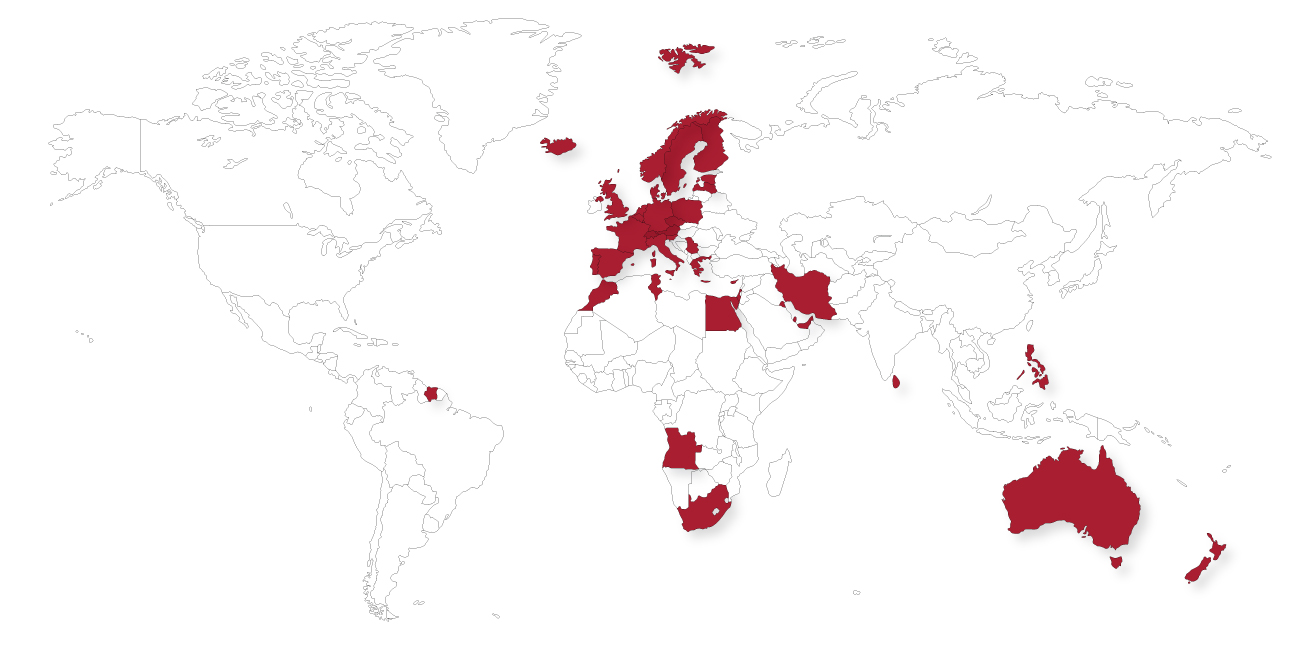
OUR FOOTPRINT
Our logistics facilities are fully compliant with GxP certificates. As a result, it enables us to distribute clinical trial supplies, biotechnology clinical trial and authorised human medicines worldwide.
Over time, Eumedica has built a broad distribution network. Therefore, it ensures access to a product portfolio in the United Kingdom, France and BeNeLux.
Key Facts & Figures
- Over 70,000 patients cared for every year
- 18 products covering 6 niche therapeutic areas
- More than 35 years of experience